 |
| Define Indicator -Class 10 |
Indicator is most important topic of NCERT Acids bases salt chapter 2
in Class 10. Questions are
frequently asked in the CBSE board and
ICSE Board exam from definition
of indicator .
“Chemistry – Define
Indicator : class 10 notes “ will be
very beneficial for the students who are engaged in the preparation of upcoming board exam.
In this topic, the following terms will be
illustrated.
Contents :
* 1. Definition of Indicator
* 2. Type of Indicator : Natural Indicator , Synthetic Indicators , Olfactory Indicator , Universal Indicator
* 3. pH scale color chart , Importance of pH in our life
Definition of indicator :
- A substance which is used to detect acidic or basic medium is called indicator.
- When indicator is kept in acidic or basic medium , its colour or smell is changed.
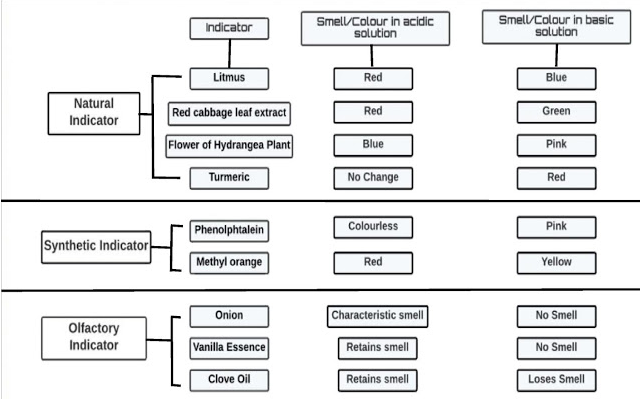
Types of indicators:
1. Natural Indicator:
- It occurs naturally. e.g Litmus, Turmeric, Red cabbage etc.
- It is natural indicator whose neutral colour is purple.
- It is extracted from lichen .
- It is used in form of liquid as well as paper strip.
- It turns red in acidic medium and blue in basic medium.
- It is also neutral indicator.
- It is yellow in neutral condition.
- It remains same in acidic medium.
- It turns red in basic medium.
- Its neutral colour is red.
- It remains red in acidic medium.
- It turns green in basic medium.
NATURAL INDICATOR | |||
Indicator | Neutral Colour | Colour In acidic Medium | Colour In Basic Medium |
Litmus | Purple | Red | Blue |
Turmeric | Yellow | Yellow | Red |
Red cabbage | Red | Red | Green |
2. Synthetic Indicators:
- The indicators which are prepared in laboratory are called synthetic indicators.
 |
| Structure of Methyl orange |
- Its neutral colour is orange.
- It turn red in acidic medium.
- It turns yellow in basic medium.
 |
| Phenolphthalein |
- It is colorless in neutral state.
- It remains colorless in acidic medium.
- It turns pink in basic medium
Synthetic Indicaror | Neutral Colour | Colour In acidic Medium | Colour In Basic Medium |
Methyl orange | Orange | Red | Yellow |
Phenolphthalein | Colourless | Colorless | Pink |
3. Olfactory Indicator:
- Those substances whose smell change in acidic or basic medium are called olfactory indicators.
- The term ‘ olfactory ‘ means ‘ relating to the sense of smell’ .
- Onion and vanilla extract are olfactory indicators.
- Characteristics smell of onion and vanilla are remain same in acidic medium .
- Characteristics smell of onion and vanilla can not be detected in basic medium.
4. Universal Indicator:
- Those substance which is used to detect acidic and basic medium with strength of Hydrogen ions (pH ) is called universal indicator.
- Universal indicator is a mixture of many different indicators which gives different color at different pH value of medium.
- The colour produced by universal indicator when mixed with given solution is used to find pH value of solution by matching the colour with the colour on pH colour chart.
ph scale color chart | ||
Colour of solution | pH Value | Nature of medium |
Dark red | 0 | Acidic Medium |
Red | 1 | |
Red (pink ) | 2 | |
Orange red | 3 | |
Orange | 4 | |
Orange yellow | 5 | |
Greenish yellow | 6 | |
Green | 7 | Neutral Medium |
Greenish blue | 8 | Basic Medium |
Blue | 9 | |
Navy blue | 10 | |
Purple | 11 | |
Dark purple | 12 | |
Violet | 13 | |
Dark violet | 14 | |
Importance of pH in Everyday Life
Our body works within the pH range of 7.0 to 7.8. Living organisms can survive only in a narrow
range of pH change. When pH of rain
water is less than 5.6, it is called acid rain. When acid rain flows into the
rivers, it lowers the pH of the river water. The survival of aquatic life in
such rivers becomes difficult.
pH in our digestive system
* Our stomach produces hydrochloric acid. It helps in the digestion
of food without harming the stomach.
* During indigestion the stomach produces too much acid and
this causes pain and irritation.
* To get rid of this pain, we use bases called antacids. These antacids neutralize the excess of acid .
* Milk of magnesia
Is often used for this purpose. Chemical name of Milk of magnesia is Magnesium hydroxide Mg (OH)2 .
pH change as the cause of tooth decay
* Tooth decay starts when the pH of the mouth is lower than
5.5.
* Tooth enamel, made up of calcium hydroxyapatite. calcium hydroxyapatite is a crystalline form
of calcium phosphate which is the
hardest substance In the body.
* Bacteria present in the mouth produce acids by degradation
of sugar and food particles remaining In the mouth after eating.
* It is corroded when
the pH in the mouth is below 5.5.
* The best way to prevent this is to clean the mouth after
eating food.
* Using toothpastes, which are generally basic, for cleaning
the teeth can neutralize the excess acid and prevent tooth decay.
Self defence by animals and plants through chemical warfare
* Bee-sting leaves an acid which causes pain and Irritation.
* Use of a mild base like baking soda on the stung area gives
relief.
* Stinging hair of nettle leaves inject methanol acid causing
burning pain.
If you liked the notes of Indicator, then share
it with other students.
If you want to ask any question related to topic definition
of indicator then write a question in the comment box.
Related Article ( Must read )
* What are Acid and Its Chemical Properties
* Base and Alkali – Chemistry Notes Class 10
* What is Salt – Chemistry Notes Class 10
* Acid Base Salt - common name

No comments:
Post a Comment