Base definition and its properties

Definition of base and its properties is most important topic of NCERT Acids bases salt chapter 2 in Class 10. Questions are frequently asked in the CBSE board and ICSE Board exam from bases .
“Chemistry – Define
base : class 10 notes “ will be very beneficial for the students who are
engaged in the preparation of upcoming
board exam.
In this topic, the following terms will be
illustrated.
Contents :
* 1. Definition of base
* 2. What are alkalis
* 3. Physical Properties of base
* 4. Chemical Reactions of base
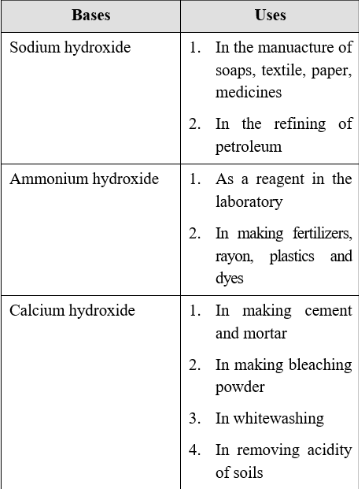
* 5. Uses of common Bases
Base definition
- Base are the substance which neutralize the acid to form salt and water only .
- Metal oxide
- Metal hydroxide
- Those substances which dissociate into hydroxide ions in aqueous solution are called base.
- Water soluble bases are also known as alkalis.
- Strong Alkalis : NaOH, KOH
- Moderately strong alkalis: Mg(OH)2, Ca(OH)2, Ba(OH)2
- Weak alkalis : NH4OH
- Soluble bases are much more useful than insoluble bases because most of chemical reaction take place only in aqueous solution.
Strong alkali:
- A base whose all molecules dissociate into hydroxide ( hydroxyl) ions is called strong alkali.
- A base whose few molecules dissociate into hydroxyl ions is called moderately strong alkali.
- A base whose very few molecules dissociate into hydroxyl ions is called weak alkali.
Common Name of Alkalis
* NaOH - Caustic soda
* KOH - Caustic
potash
* Mg(OH)2 - Milk of magnesia
* NH4OH - Ammonia solution, ammonia water, aqueous
ammonia, or aqua ammonia
* Ca(OH)2 - slaked lime
Note : Limewater is the common name for a saturated solution
of calcium hydroxide.
Properties of Bases:
Physical Properties:
- Bases have bitter tase.
- Bases feel soapy to touch.
- Bases turn red litmus blue.
- Bases conduct electricity in aqueous solution. i.e bases are electrolyte.
Chemical Reactions:
Alkalis NaOH, KOH | + Metal → | Salt + H2 |
+ Acid → | Salt + H2O | |
+ Non-Metal Oxide → | Salt + H2O |
Reaction With Some Metals:
- Bases react with some metals like Zn, Al, Pb, Sn to form salt and hydrogen gas.
- Base react with acid to form salt and water.
- Base react with Non-metal oxide to form salt and water.
- It is used as antacid to neutralize excess acid in the stomach.
- It is called milk of magnesia.
- It is used as washing soda.
- It is used for softening hard water.
- It is used as baking soda in cooking.
- It is used for making baking powder.
- It is used as antacid.
- It is used in fire extinguisher.
If you liked the notes of Alkali Base definition and its properties, then share it with other students.
If you want to ask any question related to topic Base class X notes then
write a question in the comment box.
Related Article ( Must read )
* What are Acid and Its Chemical Properties
* Define Indicator in Chemistry –Notes Class 10
* What is Salt – Chemistry Notes Class 10
* Acid Base Salt - common name

No comments:
Post a Comment