In this chapter we will discuss about corrosion , rusting of iron , condition for rusting. its prevention. corrosion of copper, aluminium, silver. In this topic we will also discuss about alloy and its constituents.
 |
| Rusting of iron |
Corrosion:
The formation of dull layer on metallic surface in presence of air, water or other chemical is called corrosion.
Rusting :
- The corrosion of iron is called rusting.
- When an iron object is kept in damp air (or water) for a considerable time, it gets covered with a red- brown flaky substance called rust. This process is called rusting of iron.
- During the rusting of iron, iron metal combines with the oxygen of air in the presence of water to form hydrated iron (II) oxide, Fe2O3.xH2O .
- This hydrated iron (II) oxide is called rust.
- So, rust is mainly hydrated iron (II) oxide, Fe2O3.xH2O (the number of molecules of water x varies, it is not fixed).
Necessary Conditions for the Rusting of Iron:
- Two conditions are necessary for the rusting of iron to take place:
1. Presence of air (or oxygen)
2. Presence of water (or moisture)
Prevention of Rusting :
- The various common methods of preventing the rusting of iron are given below:
Rusting of iron can be prevented by painting.
- The most common method of preventing the rusting of iron is to coat its surface with a paint.When a coat of paint is applied to the surface of an iron object, then air and moisture cannot come in contact with the iron object and hence no rusting takes place.
Rusting of iron can be prevented by applying grease or oil.
- When some grease or oil is applied to the surface of an iron object, then air and moisture çan not come in contact with it and hence rusting is prevented.
Rusting of iron can be prevented by galvanisation.
- The process of depositing a thin layer of zinc metal on iron objects is called galvanisation.
- Galvanisation is done by dipping an iron object in molten zinc metal.
- Zinc is a quite reactive metal. The action of air on zinc metal forms a very thin coating of zinc oxide all over it. This zinc oxide coating is hard and impervious to air and hence prevents the further corrosion of zinc metal .
Rusting of iron can be prevented by tin-plating and chromium-plating.
- Tin and chromium metals are resistant to corrosion. So, when a thin layer of tin metal (or chromium metal) is deposited on iron and steel objects by electroplating, then the iron and steel objects are protected from rusting. For example, tiffin-boxes made of'steel are nickel-plated from inside and outside to protect them from rusting.
- Tin is used for plating tiffin-boxes because it is non-poisonous and hence does not contaminate the food kept in them.
- Chromium-plating is done on taps, bicycle handle bars and car bumpers made of iron and steel to protect them from rusting and give them a shiny appearance.
Rusting of iron can be prevented by alloying it.
- E.g : When iron is alloyed with chromium and nickel, then stainless steel is obtained. Stainless steel does not rust at all. Cooking utensils, knives, scissors and surgical instruments, etc., are made of stainless steel and do not rust at all.
Corrosion of Aluminium Metal :
- The action of moist air on aluminium metal forms a thin layer of aluminium oxide all over the aluminium metal.
- Due to the formation of a dull layer of aluminium oxide , the aluminium loses its shine very soon.
- This thin aluminium oxide layer is very tough and prevents the metal underneath from further corrosion .
- If the aluminium oxide layer on the surface of aluminium objects could somehow be made thicker, then the aluminium objects would be protected from corrosion even more effectively. This can be done by a process called 'anodising
Anodising:
- Anodising is a process of forming a thick layer of aluminium oxide on an aluminium object by making it anode in electrolysis .
- The layer of aluminium oxide on the surface of aluminium objects can be made thicker by electrolysis. This process is called anodising.
- In this process, the aluminium object is made an anode (positive electrode) in an electrolytic tank in which dilute sulphuric acid is electrolysed. During the electrolysis of dilute sulphuric acid, oxygen gas is liberated at the anode and reacts with the aluminium object to form a thicker layer of aluminium oxide on its surface.
- This thicker and more uniform aluminium oxide layer protects the aluminium object from corrosion very effectively.
Corrosion Copper:
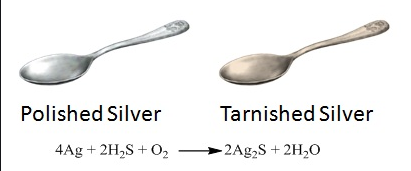
- Silver is a highly unreactive metal so it does not react with the oxygen of air easily. But air usually contains a little of sulphur compounds such as hydrogen sulphide gas (H2S).
- So, the silver objects combine slowly with the hydrogen sulphide gas present in air to form a black coating of silver sulphide (Ag2S).
- The shining silver objects become tarnished due to the formation of silver sulphide coating on their, surface. Thus, silver ornaments gradually turn black due to the formation of a thin layer of silver sulphide.
- An alloy is a homogeneous mixture of two or more elements in which major component are metals.
For example,
- Brass is an alloy of two metals : copper and zinc.
- Steel is an alloy of a metal and a small amount of a non- metal: iron and carbon,
- An alloy is prepared by mixing the various metals in molten state in required proportions and then cooling their mixture to the room temperature.
- The alloy of a metal and a non-metal can be prepared by first melting the metal and then dissolving the non-metal in it, followed by cooling to the room temperature.
- Each alloy has certain useful properties. The properties of an alloy are different from the properties of the constituent metals.
Characteristics of Alloy:
- Alloys are stronger than its constituent metals.
- Alloys are harder than the constituent metals.
- Alloys are more resistant to corrosion.
- Alloys have lower melting points than the constituent metals.
- Alloys have lower electrical conductivity than constituent metals.

No comments:
Post a Comment