 |
ALCOHOLS : |
- Alcohols are the organic compounds whose molecules consist hydroxyl functional group (-OH group) attached to a carbon atom .
- Thus an alcohol is hydroxyl derivative of an alkane.
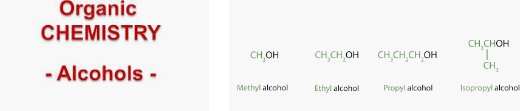
- The general formula of the homologous series of alcohols is CnH2n+1-OH, where ‘n’ is the number of carbon atoms in one molecule of the alcohol. For example, if the number of carbon atoms in an alcohol is one, the formula of this alcohol will be CH3-OH.
- The two simple alcohols are methyl alcohol, CH3OH (methanol) and ethyl alcohol C2H5OH (ethanol).
IUPAC Nomenclature Of Alcohol Family:
- IUPAC name of Alcohol may consist three part.
- Branch name , root name and family name ( anol )
- It is prefix of alcohol name .
- Branch is alkyl group or any substitution
- Branch name is taken according to its position on root.
- If there are more than two branch in alcohol , then proper rule is followed in naming.
- It comes after branch name.
- It depend on number of carbon atoms present in root of molecules.
- Family name of alcohol is anol . and suffix is ol .
- Step -1 : Determine root of alcohol molecule .
- Step -2: Count number of carbons present in root and then decide its root name.
- Step -3 :Give number the carbon atoms of root from that end so that --OH group gets lowest possible number.
- Name the compound in proper order: branch name, root name, family name.
Example :
Naming of CH3OH
Root name : meth
Family name : anol
So IUPAC name : methanol
Common name: Methyl Alcohol
Naming of C2H5OH:
CH3 ----- CH2--OH
Root name : eth
Family name : anol
So IUPAC name : ethanol
Common name : Ethyl alcohol
Naming of C3H7OH:
CH3 --- CH2 --- CH2--OH
Root name : prop
Family name : anol
So IUPAC name : propanol
Common name : n-propyl alcohol
Naming of C4H9OH:
CH3 --- CH2 ---CH2—CH2---OH
Root name : but
Family name : anol
So IUPAC name : butanol
Common name : n-butyl alcohol
Some Important Alcohol:
- Ethanol is the second member of alcohols family. Its common name is ethyl alcohol.
- The formula of ethanol is C2H5OH which can also be written as: CH3-CH2OH .
- It is most common alcohol and widely used . so ethanol is also called just alcohol.
Physical Properties Of Ethanol :
- Ethanol is a colourless liquid having a pleasant smell and a burning taste.
- Ethanol is a volatile liquid having a low boiling point of 78°C (351 K).
- It is lighter than water.
- Ethanol mixes with water in any proportion. The solubility of ethanol in water is due to the presence of hydroxyl group in it.
Rectified Spirit:
- Ethanol containing 5 % water is called rectified spirit.
- Rectified spirit is the commercial alcohol.
,:
- 100 % pure ethanol is called absolute alcohol.
Chemical Properties of Ethanol :
- Ethanol is a covalent compound. Ethanol does not contain any hydrogen ions, so it is a neutral compound. Thus, ethanol has no effect on any litmus solution.
- The chemical properties of ethanol are following types.
Combustion:
- Ethanol is a highly inflammable liquid.
- Ethanol burns readily in oxygen to form carbon dioxide and water. In this reaction a lot of heat and light are released .
- Since ethanol burns with clear flame giving a lot of heat, therefore, it is used as a fuel.
Some terms:
Alkaline Potassium Permanganate Solution:
- An aqueous solution of potassium permanganate containing sodium hydroxide is called alkaline potassium permanganate solution.
- Thus alkaline potassium permanganate solution is KMnO4 + NaOH.
Acidified Potassium Dichromate Solution:
- The potassium dichromate solution containing sulphuric acid is called acidified potassium dichromate solution.
- Thus acidified potassium dichromate solution is K2Cr2O7 + H2SO4.
- Alkaline potassium permanganate and acidified potassium dichromate are strong oxidising agents (because they provide oxygen for oxidising other substances).
Nascent Oxygen:
- Nascent oxygen is freshly generated atomic oxygen which is very, very reactive.
Oxidation:
- When ethanol is heated with alkaline potassium permanganate solution (or acidified potassium dichromate solution), it gets oxidized to ethanoic acid.
- Thus, ethanoic acid is formed by the oxidation of ethanol by using a strong oxidising agent.
- This ethanoic acid turns blue litmus to red.
Reaction With Sodium Metal:
- Ethanol reacts with sodium to produce sodium ethoxide and evolve hydrogen gas.
- All member of alcohol react with sodium to produce hydrogen gas.
Test for an Ethanol:
- When a small piece of sodium metal is put into ethanol in dry test tube , hydrogen gas is evolved.
- When a lighted candle is brought near hydrogen , a pop sound is produced . pop sound is characteristics of burning hydrogen.
- This reaction is carried out for test of Alcohol.
Dehydration:
- Removal of water molecules from Alcohol is called dehydration of alcohol.
- When ethanol is heated with excess of concentrated sulphuric acid at 1700 C then it get dehydrated to form ethene.
- Concentrated H2SO4 acts as a dehydrating agent.
Reaction With Ethanoic Acid:
- Ethanol reacts on heating with ethanoic acid in presence of few drops of concentrated H2SO4 to form ester.
- This reaction is called esterification.
- In this reaction , ester is formed which has sweet smell.
- So this reaction is carried out for test of Alcohol.
Uses of Ethanol (Ethyl Alcohol) :
- Ethyl alcohol (ethanol) is used in the manufacture of paints, varnishes, medicines, perfumes, dyes, soaps and synthetic rubber.
- Ethyl alcohol (ethanol) is used as a solvent. Many organic compounds which are insoluble in water, are soluble in ethyl alcohol.
- Being a good solvent, ethyl alcohol (ethanol) is used in medicines such as tincture iodine, cough syrups and many tonics.
- Ethyl alcohol (ethanol) is used as a fuel in cars along with petrol. It is also used as a fuel in spirit lamps.
- Ethyl alcohol (ethanol) is used in alcoholic drinks (beverages) like whisky, wine, beer and other liquors.
Whisky contains about 35% of ethyl alcohol, wine contains 10% to 20% of ethyl alcohol, and beer contains about 6 % of ethyl alcohol.
- Ethyl alcohol (ethanol) is used as an antiseptic to sterilize wounds and syringes in hospitals and dispensaries.




No comments:
Post a Comment